What are Clean-in-Place Seals?
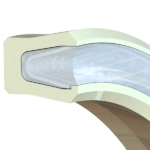
Clean in place seals, or CIP seals, were developed to allow a seal to remain in place. This is especially important when the seal gland is partially open, allowing the seal to be flushed of debris.
In a food application , the same chemicals used to clean or flush the system would be used to clean the seal gland. Similarly, when other products such as pharmaceutical or adverse chemicals needed to be flushed, the CIP seal does an excellent job being open to flushing.
CIP Seal Designs and Materials
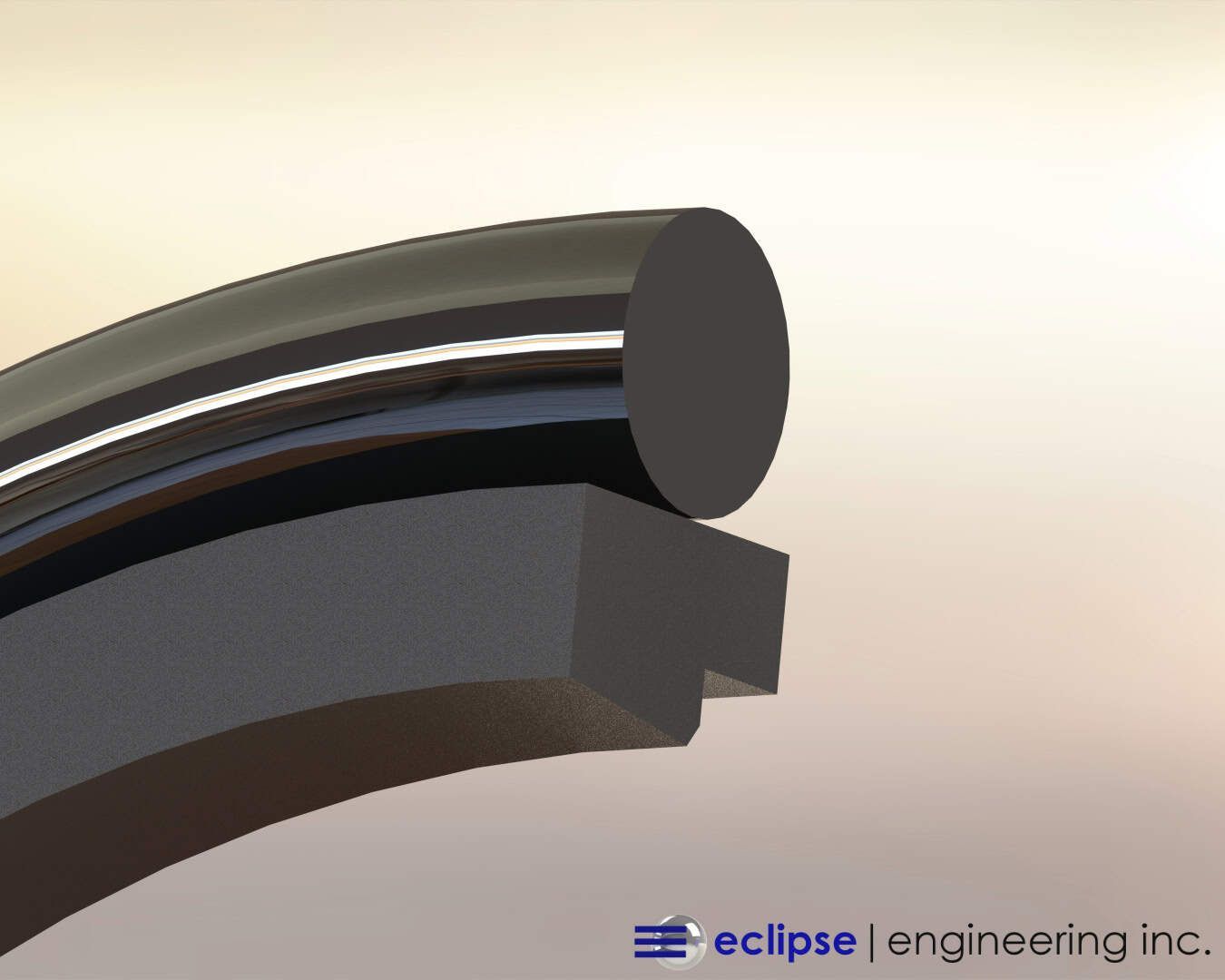
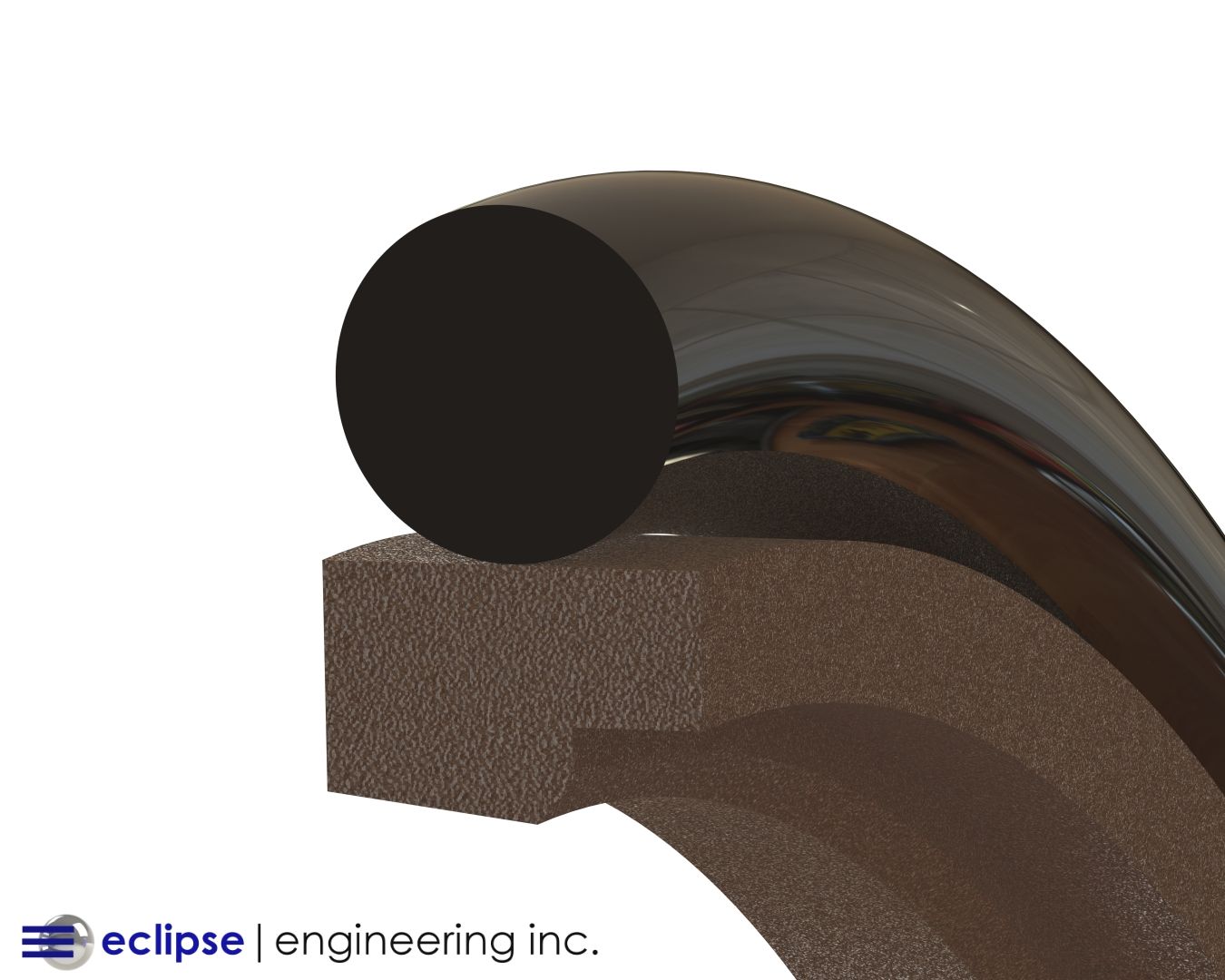
There are several styles of seals that are often found in these applications. Spring energized seals are frequently used because with this type of seal, the cantilever spring and seal groove can be filled with silicone. The silicone fills the void in the seal cavity and covers the spring, allowing the seal to be flushed.
Another typical design would be a simple lip seal without a spring. The lip can face in either direction. For CIP seals, it’s best to have the open side face the product so that during a cleaning, the complete seal is exposed. This ensures that all debris will be flushed out.
Another advantage to using either Teflon or canned lip seals is that these materials will normally be compatible with any cleaning solutions you use to flush the seal cavity.
CIP Seals and FDA Compliance
When designing for food or drug applications where the seal will be flushed, Eclipse Engineering can design with materials that may be FDA compliant both in the environment and the cleaning fluid used to flush the gland free of debris.
In special cases, double lip seals with lips facing axially in forward and reverse direction can be machined, spring energized, and filled with silicone.
Piston and Plunger CIP Seals
Eclipse also builds and integrates a piston design which has the seal built into the piston. We install a spring in the seal and use silicone to completely occupy the spring groove. This design ensures that no particles can become trapped in the seal groove, as it’s completely integrated into the design.
Piston or plunger designs for filling often take advantage of this integrated design. A typical application could be filling a cup with pudding or some other viscous media.
We often use UHMW, or Ultra High Molecular Weight Polyethylene
, for its low-friction, FDA compliance, and ease of cleaning.
There are some cases where the seal is used for one type of product and discarded prior to switching to a different fill. In those cases, ease of removal into stepped glands ensures the seal can be replaced without a gland keeper, which could trap particles and contaminate a batch.
Usually upon any disassembly, a CIP seal is damaged and can no longer be cleaned. This is because the retention devices used are one piece and part of the gland, and are easily flushed with particulate.
The success or failure of a seal design is often dependent on how the seal was installed. There are many seals that easily slip into glands with simple installation procedures. But when installing seals with any volume, it’s important to have an established method to ensure consistent performance of the seal.